High-performance Na-ion battery cathodes and anodes
Students involved:
PhD students: Jintao Fu, Manni Li, Sam Welborn, John Corsi, Zeyu Wang
Master’s degree students: Yifeng Zheng
Undergraduate students: Olivia Ruiz
Recently sodium-ion batteries (SIBs) have been the subject of intense research as an alternative to lithium-ion battery (LIB) for large-scale energy storage applications. As a matter of fact, a UK-based company called Faradion has started to manufacture SIBs for low-cost electric transports including low-speed electric vehicles, e-scooters and e-bikes. Our group develops earth-abundant 3D nanoporous materials for SIB anode and cathodes. Ongoing projects include:
- High-rate and long cycle-life of bulk Na2/3[Ni1/3Mn2/3]O2 sodium-ion battery cathode enable through inclusion
Among various candidate cathodes materials for SIBs, the layered oxides NaTMO2 (TM=3d transition metal elements and their mixture) are very attractive as high-voltage cathodes. However, they suffer from a poor rate capability and short cycle-life. This project is aimed at using non-precious earth-abundant 3D nanoporous metal scaffolds in combination with solid-state reactions, to develop a novel class of bulk Na2/3[Ni1/3Mn2/3]O2 with structural inclusions for high-performance SIBs cathodes. In the bulk form, this exhibits Na2/3[Ni1/3Mn2/3]O2 an unprecedented rate capability with roughly 50 % capacity retention at 50 C, and a remarkable long-term cycling stability, with excellent capacity retention after a few thousands cycles.Typical galvanostatic curves and cycling stability at the rate of 5 C are shown in Figure 1. From Figure 1(a) it can be seen that the redox plateaus are clearly displayed, even at higher C-rates, i.e. 5 C. On the other hand, the long-term cycling stability shown in Figure 1(b) cycled at 5C preserves over 80% capacity retention after 1200 cycles. Such performance indicates the superior electrode kinetics, probably due to the increase in insertion layer distance enabled through structural (void) inclusions.
Reference: J. Fu, J. Corsi, M. Li, Y. Zheng and E. Detsi, Unpublished
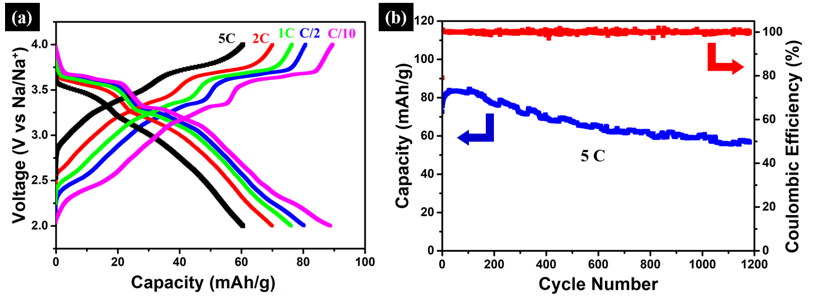 Figure 1: (a) Rate-capability of the Na2/3[Ni1/3Mn2/3]O2 cathode materials for SIB. Even at 5C the redox plateau is still clearly displayed. (b) Long-term cycling stability of the Na2/3[Ni1/3Mn2/3]O2 cathode materials cycled at 5C. Even at this ultra-fast C-rate, over 80% capacity retention was achieved after 1200 cycles. Such performance indicates the superior electrode kinetics, due to the increase in insertion layer distance enabled through structural (void) inclusions.
Figure 1: (a) Rate-capability of the Na2/3[Ni1/3Mn2/3]O2 cathode materials for SIB. Even at 5C the redox plateau is still clearly displayed. (b) Long-term cycling stability of the Na2/3[Ni1/3Mn2/3]O2 cathode materials cycled at 5C. Even at this ultra-fast C-rate, over 80% capacity retention was achieved after 1200 cycles. Such performance indicates the superior electrode kinetics, due to the increase in insertion layer distance enabled through structural (void) inclusions.- Bulk nanoporousactive/inactive composites as high-performance alloy-type Na-ion battery anodes
Engineering approaches based on “nanostructuring” and “dense active/inactive composites” are commonly used separately to incrementally improve the performance of alkali-ion battery anodes. This project is aimed at significantly improving the performance of alloy-type Na-ion battery anodes by merging these two engineering approaches to make nanostructured active/inactive composites, consisting of a nanoporous phase, used as the electrochemical active component, and a dense phase, used as the inactive component and acting as a mechanical buffer.
Reference: O. Ruiz, M. Cochrane, M. Li, Y. Yan, K. Ma, J. Fu, Z. Wang, S.H. Tolbert, V.B. Shenoy and E. Detsi, Advanced Energy Materials 8, 1801781, 2018
A typical configuration of such a nanoporous active/inactive composite is shown in Figure 2, with nanoporous Sb particles (in red) used as the electrochemically-active phase for Na storage (leading to the formation of nanoporous Na3Sb), and dense magnesium fluoride (MgF2) particles (in blue) used as the electrochemically-inert phase, which acts as a mechanical buffer. Figure 3 illustrates the typical performance of nanoporous Sb/MgF2 active/inactive composites during Na storage; the green and red curves represent the capacity as a function of the number of cycles for two porous Sb/MgF2 composite samples charged and discharged in two hours (corresponding to a C-rate of C/2) and in one hour (corresponding to a C-rate of 1 C), respectively. They both underwent over 300 charging/discharging cycles with high capacity retention, while the commercial Sb sample cycled at C/2 underwent full capacity fading within the first 20 cycles (blue curve Figure 3). After 300 cycles, the capacity retained in the porous Sb/MgF2 anode cycled at 1 C is 430 mAh/g, which is equivalent to ~65% of the theoretical capacity of Sb.
This project involves a collaboration between 3DAFSN Detsi Research Lab (Penn/MSE), Stach Lab (Penn/MSE) and Rappe Lab (Penn Chemistry). This project is currently supported through a Seed Grant from the Vagelos Institute for Energy Science and Technology (VIEST).
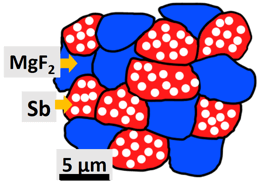
Figure 2: Illustration of nanoporous Sb/MgF2 active/inactive composite SIB anode made of electro-chemically-active nanoporous Sb particles (red), and inactive dense MgF2 particles (blue). Reference: O. Ruiz, M. Cochrane, M. Li, Y. Yan, K. Ma, J. Fu, Z. Wang, S.H. Tolbert, V.B. Shenoy and E. Detsi, Advanced Energy Materials 8, 1801781, 2018
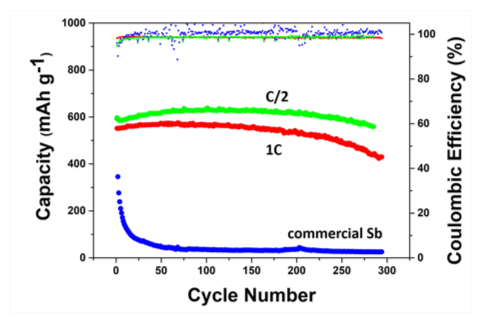
Figure 3: Capacity and Coulombic efficiency as a function of the number of cycles for porous Sb/MgF2 (green & red curves), and commercial bulk Sb (blue curve). Reference: O. Ruiz, M. Cochrane, M. Li, Y. Yan, K. Ma, J. Fu, Z. Wang, S.H. Tolbert, V.B. Shenoy and E. Detsi, Advanced Energy Materials 8, 1801781, 2018