High-performance Mg-ion battery anodes
Students involved:
PhD students: Lin Wang, Sam Welborn, John Corsi, Zeyu Wang, Manni Li
Undergraduate students: Alexander Proschel
Research assistant: Dongyang Zhang
Over the past few years, magnesium-ion batteries (MIBs) have been the subject of intense research as an alternative to lithium, owing to the various advantages of magnesium including the abundance of magnesium resources for large-scale applications. Nevertheless, progress toward practical MIBs has been impeded, partly by the absence of suitable electrolytes that are compatible with magnesium metal used as the negative electrode. Typically, common salts and organic solvents combinations similar to those used in lithium-ion batteries yield a magnesium-ion-blocking passive film on the magnesium metal negative electrode. A promising way to circumvent this electrolyte issue is through the use of alloy-type anodes as the negative electrode instead of magnesium metal. However, state-of-the-art alloy-type magnesium-ion battery anodes can only be reversibly cycled up to 200 times with acceptable capacity retention [1]. Such a cycle-life is far below the thousands of cycles required for practical battery applications. Our group develops high-performance MIB anodes from earth-abundant materials. Below are some ongoing projects.
[1] Reference: Cheng et al: Adv. Mater. 27, 6598-6605
- Long cycle-Life and high-rate Mg-ion battery anodes enabled by self-healing through near-room-temperature solid-liquid phase transition
This project is aimed at developing a novel class of high-performance alloy-type magnesium-ion battery anodes that can be reversibly (de)magnesiated at very high C-rates over 1000 cycles with excellent capacity retention. To do so, we take advantage of the self-healing property of the active electrode material, occurring through solid-to-liquid phase transition when the cell operates near-room-temperatures or below. Operando X-ray scattering techniques are used to study this self-healing property in real-time during magnesiation and demagnesiation.
Reference: L. Wang, S.S. Welborn, H. Kumar, Z. Wang, M. Li, V. Shenoy, and E. Detsi, Unpublished
- Scalable processing route to oxide-free alloy-type Mg-ion battery anodes
Nanostructured materials used as alloy-type anodes in LIBs are commonly covered with a native oxide shell formed during their processing. While this oxide shell does not hinder the storage of Li or Na in LIB and SIB batteries, in MIBs however, the diffusivity of divalent Mg ions through such a native oxide is restricted, making it difficult to reversibly store Mg in common alloy-type nanostructured anode materials such as Sn, Si and Al at practical rates. In fact, to our knowledge, the reversible Mg storage in nanostructured Si has not been experimentally demonstrated, although theoretical studies predict that these reactions are thermodynamically favorable. This project is aimed at developing scalable air-free processing routes to nanostructured materials without native oxide coverage, for use as high-performance Mg-ion battery anodes. We have successfully fabricated oxide-free nanostructured Sn and demonstrated its use as a high-performance Mg-ion battery anode [1]. We have also demonstrated the processing of oxide-free nanostructured Si for use as ultrahigh capacity Mg-ion battery anodes. Our current protocol is illustrated in Figure 1 below. This project is supported through NSF-EAGER program with award number: CMMI #1840672.
[1] Reference: H. Yaghoobnejad Asl, J. Fu, H. Kumar, S.S. Welborn, V.B. Shenoy and E. Detsi, Chemistry of Materials 30, 1815-1824, (2018)
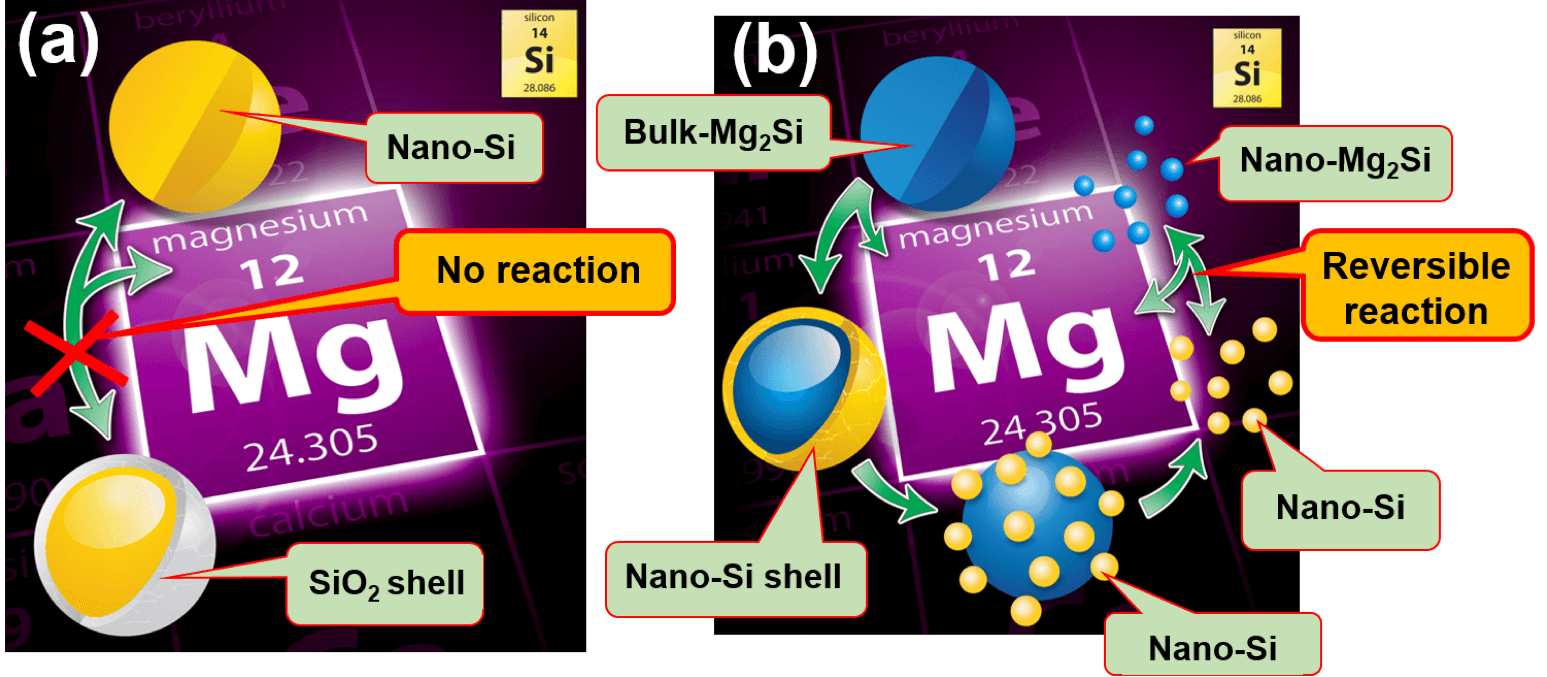 Figure 1: Graphical illustration of Mg storage in nanostructured Si (nano-Si).
Figure 1: Graphical illustration of Mg storage in nanostructured Si (nano-Si).(a) The yellow particle is a nano-Si covered with its native oxide –SiO2 (the light-grey SiO2 shell around the yellow nano-Si core). This oxide shell restricts Mg storage in nano-Si (b) Our research: instead of using nano-Si coated with its native oxide as the anode in MIB, we use micrometer-sized bulk parent materials as illustrated by the blue Mg2Si particle. The bulk Mg2Si particles will be converted into nano-Si inside the battery, when the cell is operating (i.e. in situ processing), as depicted by the yellow nano-Si shell around the blue and yellow Si nanoparticles. In this manner, the freshly made nano-Si is oxide-free because the battery cell operates under inert environment. Reference: D. Zhang, J. Corsi, J. Fu, Z. Wang and E. Detsi, Unpublished