In situ and Operando X-ray Scattering
Studies of Electrochemical Processes
Students involved:
PhD students: Sam Welborn, Alex Ng
Penn has recently acquired through a NSF-MRI grant (award number: DMR–1725969) a multi-detector Dual Cu/Mo Source X-Ray Scattering instrument (DEXS, see Figure 1) with small-angle to wide-angle X-ray scattering (SAXS and WAXS) capabilities for advanced materials characterization and educational [1]. The objective of this research is to use this laboratory-scale SAXS/WAXS setup in combination with in situ and operando electrochemical experiments to study electrochemical processes in real-time, such as the mechanism of nanoporosity formation during electrolytic dealloying, curvature evolution in 3D nanoporous metals during coarsening, and morphology evolution in battery electrodes during charging and discharging. Some examples of ongoing projects in the 3DAFSN Lab are provided below.
[1] Reference: S.S. Welborn and E. Detsi, Nanoscale Horizons, 2019. DOI10.1039/C9NH00347A
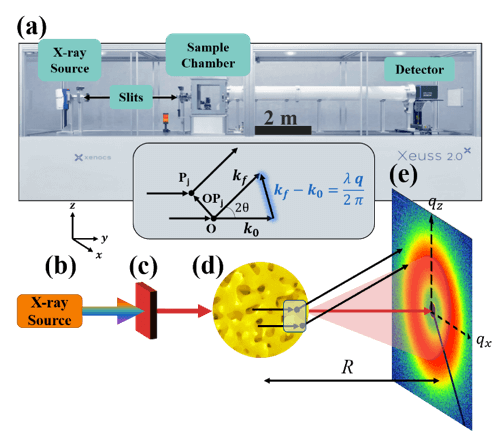
Figure 1: (a) Penn lab-scale SAXS instrument.
X-rays are generated at point (b) and are monochromated and collimated at point (c). The beam bombards the material at point (d), where the X-rays do one of three things: scatter with the electrons in the material, transmit through the material, or absorb into the material. The transmitted beam and secondary scattered waves travel down an evacuated tube of length R, and are detected at point (e).
Lead Student: Sam Welborn
Reference: S.S. Welborn and E. Detsi, Nanoscale Horizons, 2019. DOI10.1039/C9NH00347A
- In situ SAXS and WAXS studies of morphology evolution in 3D nanoporous metals during electrolytic dealloying
We have developed electrolytic dealloying protocols to study the interaction of X-rays with working electrode materials in real-time during electrolytic dealloying to probe the evolution of the nanoporous morphology (pore size, crystal structure, curvature) in various material systems. Our cell configuration makes it possible to simultaneously perform in situ SAXS and WAXS experiments during nanoporosity formation by selective electrolytic leaching in order to access the changes in pore size, crystal structure, and curvature. Specific tasks performed include:
- Using SAXS to investigate the pore-pore distance, and thus the characteristic structural size in nanoporous metals. The yellow pattern in Figure 2a represents a typical SAXS data obtained from nanoporous Au, where the peak centered at a q-value of ~0.11 corresponds to a pore-pore distance of ~55 nm in real space.
- Identifying suitable models to fit these curves, as shown in Figure 2a for the Debye model (dashed red), Berk model (dashed green) and Teubner-Strey (dashed green). We refer to our recent article in Nanoscale Horizons for a compilation of these models [1].
- Using these models to generate realistic structures of nanoporous Au (Figure 2b) to study their curvature (Figure 2c) and other properties such as specific surface area.
- Using X-ray scattering techniques to reveal morphology evolution of nanoporous Au in real-time during its formation by selective electrochemical removal of silver from gold-silver parent alloys [2].
Reference [1]: S.S. Welborn and E. Detsi, Nanoscale Horizons, 2019. DOI10.1039/C9NH00347A
Reference [2]: S.S. Welborn, J.S. Corsi, L. Wang, A. Lee, J. Fu, E. Detsi (In preparation)
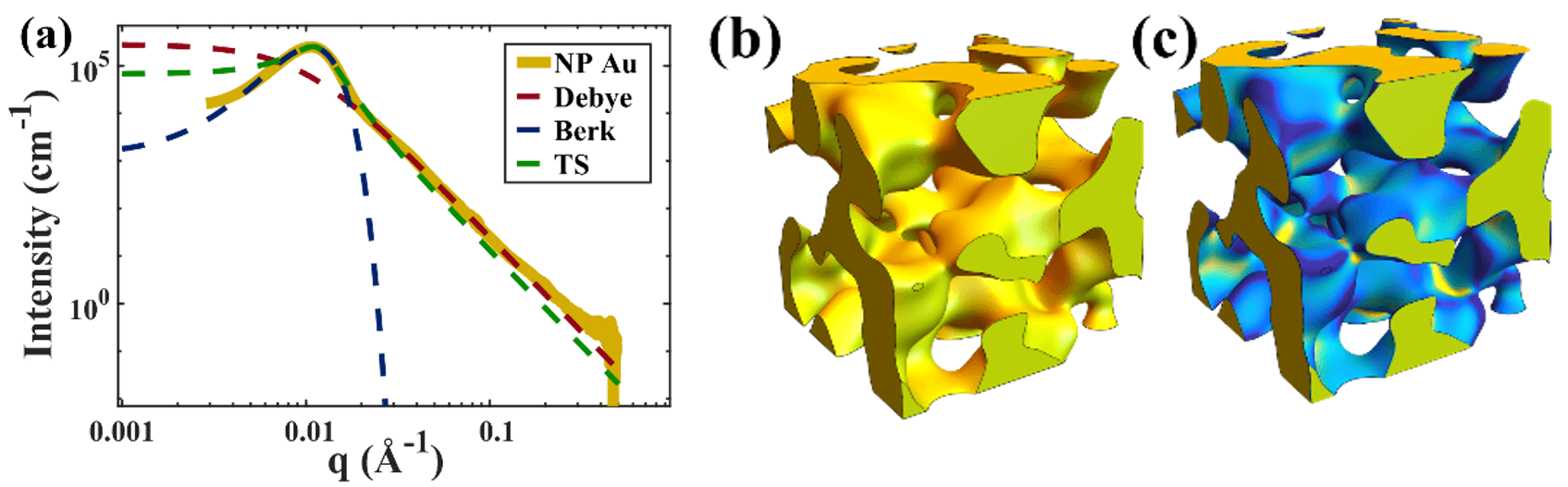 Figure 2: (a) Typical scattering intensity for NP-Au (gold curve), which we fit with various models (dashed curves). Using the fitted parameters, we can generate realistic structures of the sample (b), and calculate their curvature (c).
Figure 2: (a) Typical scattering intensity for NP-Au (gold curve), which we fit with various models (dashed curves). Using the fitted parameters, we can generate realistic structures of the sample (b), and calculate their curvature (c).Lead student: Sam Welborn
Reference: S.S. Welborn and E. Detsi, Nanoscale Horizons, 2019. DOI10.1039/C9NH00347A
- Operando SAXS and WAXS Studies of morphology evolution in battery electrodes during charging/discharging
We use X-ray scattering techniques to study the morphology (feature size and crystal structure) evolution in battery electrodes in real-time during charging and discharging. An example of ongoing project involves Na storage in nanoporous Sb used as a high-performance Na-ion battery anode. We start by fabricating 3D nanoporous Sb (see Figure 3a) by selective alloy corrosion. Next, we use electrochemical kinetics analysis methods to investigate the rate of Na storage in this nanoporous Sb to form nanoporous Na3Sb through the following reaction: Sb + (3Na++ 3e–) ←→ Na3Sb (see Figure 3b). Finally, we perform operando X-ray scattering studies, during which the crystallographic evolution of the starting nanoporous Sb is collected in real-time during reversible Na storage (i.e. charging and discharging). Figure 3c shows the typical scattering signal of Sb at 13.1° 2θ (Mo source, λ = Mo Kα). The Sb signal alternately appears and disappears during charging and discharging. The corresponding charge-discharge curves associated with the introduction (and removal) of Na in Sb (from Na3Sb) are shown in Figure 3d. The wide-angle scattering peaks of Na3Sb near 9° and 15° alternately disappear when the signal of Sb appears, and appear when the signal of Sb disappears, signifying the crystallographic phase change of this high-performance electrode.
Reference: M. Li, T. Qiu, S.S. Welborn, A.C. Foucher, B. Lesel, J. Fu, Z. Wang, D. Zhang, A.M. Rappe, E.A. Stach, E. Detsi (In preparation).
This project involves a collaboration between 3DAFSN Detsi Research Lab (Penn/MSE), Stach Lab (Penn/MSE) and Rappe Lab (Penn Chemistry). This project is currently supported through a Seed Grant from the Vagelos Institute for Energy Science and Technology (VIEST).
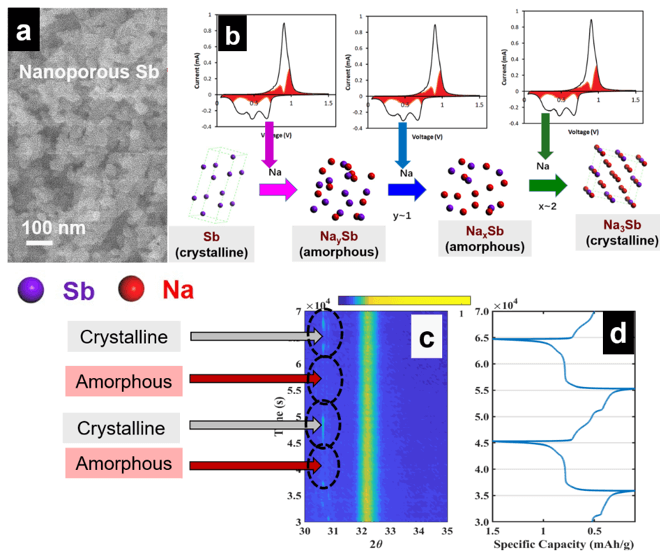 Figure 3: (a) Synthesized nanoporous Sb; (b) cyclic voltammograms obtained during kinetics analysis. The three cathodic peaks observed are associated with the insertion of three Na in Sb to make Na3Sb. During that process. (c) Real-time wide-angle X-ray scattering data during reversible Na storage in Sb; (d) corresponding electrochemical curves obtained in real-time during reversible Na storage in Sb and X-ray scattering.
Figure 3: (a) Synthesized nanoporous Sb; (b) cyclic voltammograms obtained during kinetics analysis. The three cathodic peaks observed are associated with the insertion of three Na in Sb to make Na3Sb. During that process. (c) Real-time wide-angle X-ray scattering data during reversible Na storage in Sb; (d) corresponding electrochemical curves obtained in real-time during reversible Na storage in Sb and X-ray scattering.Lead Student: Manni Li
Reference: M. Li, T. Qiu, S.S. Welborn, A.C. Foucher, B. Lesel, J. Fu, Z. Wang, D. Zhang, A.M. Rappe, E.A. Stach, E. Detsi (In preparation).
- In situ SAXS study of morphology evolution in 3D nanoporous metals during thermal and electrochemical coarsening
Dealloyed 3D nanoporous metals undergo thermally- and (electro)chemically-induced structural coarsening. We study the morphology (pore size and curvature) evolution in real-time during coarsening using X-ray scattering. Typical scattering curves from before and after thermal coarsening are shown in Figure 4a along with their corresponding SEMs in Figure 4b-c, which validate the characteristic structure size obtained from SAXS. We use a spinodal decomposition model to reconstruct the nanoporous gold morphology from the X-ray scattering data before (Figure 4d) and after coarsening (Figure 4e).
Reference: S.S. Welborn, S. van der Meer, J.S. Corsi, J.T.M. De Hosson and E. Detsi (Under review).
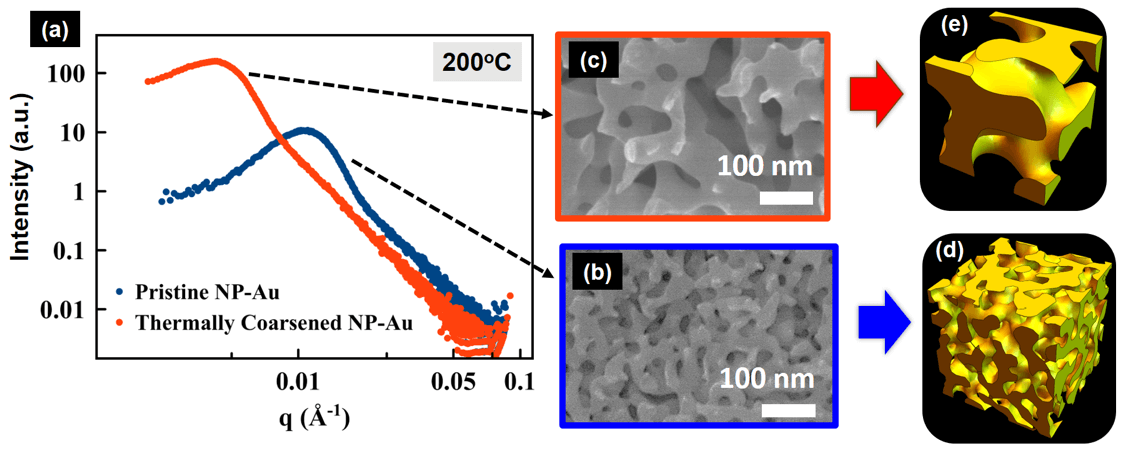 Figure 4: (a) USAXS data collected in real-time during thermal coarsening of nanoporous Au. SEM images showing the corresponding nanoporous morphology before (b) and after coarsening (c). A spinodal decomposition model was used to reconstruct the nanoporous gold morphology from the X-ray scattering data before (d) and after coarsening (e).
Figure 4: (a) USAXS data collected in real-time during thermal coarsening of nanoporous Au. SEM images showing the corresponding nanoporous morphology before (b) and after coarsening (c). A spinodal decomposition model was used to reconstruct the nanoporous gold morphology from the X-ray scattering data before (d) and after coarsening (e).Lead Student: Sam Welborn
Reference: S.S. Welborn, S. van der Meer, J.S. Corsi, J.T.M. De Hosson and E. Detsi (Under review).