In situ electrochemical dilatometry studies of battery electrodes
Students involved:
PhD students: Lin Wang, Manni Li
Master’s degree students: Ke Ma
Undergraduate students: Alexander Proschel
Phase transformations arising during Li, Na and Mg storage in Li-ion, Na-ion and Mg-ion battery electrodes, respectively, give rise to large stresses and huge volume changes in these electrodes, resulting in rapid capacity fading. In this section, we use electrochemical dilatory techniques to study these volume changes in real-time during Li, Na and Mg storage in Li-ion, Na-ion and Mg-ion battery electrodes, respectively. In addition to battery electrodes, we also use in situ electrochemical dilatometry techniques to study the volume shrinkage occurring in 3D nanoporous metals during their formation by electrochemical dealloying. Some examples of past and ongoing projects are provided below.
- Study of volume changes in battery electrodes during charging and discharging
Ongoing projects in this section involve the study of the volume changes in various battery technologies including alloy-type Mg-ion battery anodes, and alloy-type Na-ion battery anodes. In the latter case, Na storage in high-capacity alloy-type anode materials such as P (capacity 2596 mAh.g-1for the Na3P phase), Sn (capacity 847 mAh.g-1for the Na15Sn4 phase), Sb (capacity 660 mAh.g-1for the Na3Sb phase), and Ge (capacity 369 mAh.g-1for the crystalline phase NaGe; and 590 mAh.g-1for the amorphous phase Na1.6Ge) give rise to huge volume changes, which result in rapid capacity fading. We have investigated these volume changes in real-time during reversible Na storage in 3D nanoporous Ge anodes using electrochemical dilatometry techniques. Our setup and typical dilatometry data are displayed in Figure 1 below.
Reference: M. Li, Z. Wang, J. Fu, K. Ma and E. Detsi, Scripta Materialia 164, 52-56,2019
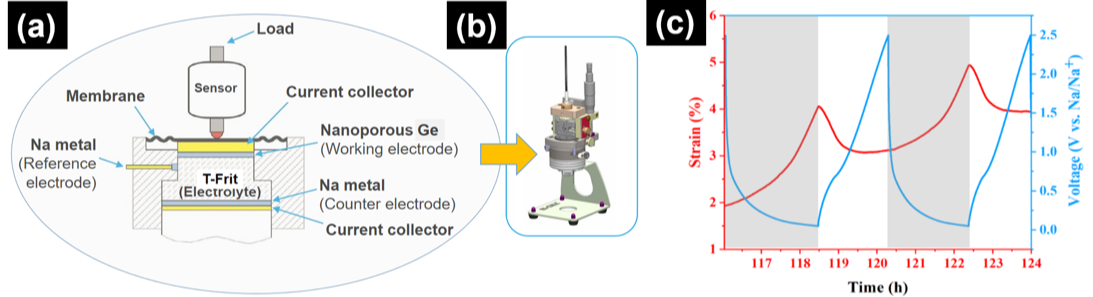 Figure 1: (a) Dilatometry setup. The strain measurement unit can be (dis)assembled in a glove box (i.e. under inert environment). (b) E-cell mounted on the dilatometer frame. (c) Measured strains in nanoporous Ge anode during charging and discharging (red) and corresponding charge/discharge curves as a function of time (blue);
Figure 1: (a) Dilatometry setup. The strain measurement unit can be (dis)assembled in a glove box (i.e. under inert environment). (b) E-cell mounted on the dilatometer frame. (c) Measured strains in nanoporous Ge anode during charging and discharging (red) and corresponding charge/discharge curves as a function of time (blue);Reference: M. Li, Z. Wang, J. Fu, K. Ma and E. Detsi, Scripta Materialia 164, 52-56,2019
- Volume shrinkage during nanoporous metals formation by dealloying
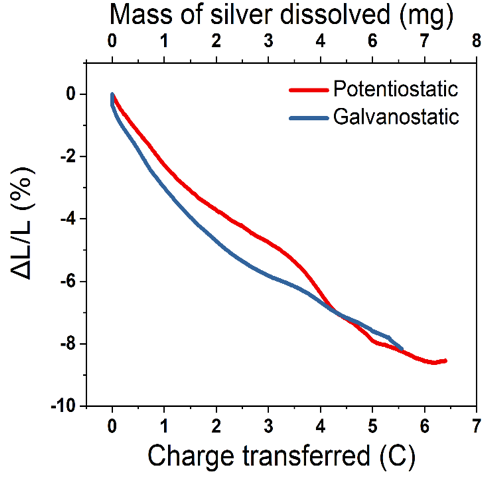 Figure 2: Linear strains versus charge transferred (bottom x-axis) and mass of dissolved silver (top x-axis) during dealloying in potentiostatic (red) and galvanostatic (blue) modes. Reference: K. Ma, J.S. Corsi, J. Fu and E. Detsi, ACS Applied Nano Materials 1, 2, 2018[/caption]
Figure 2: Linear strains versus charge transferred (bottom x-axis) and mass of dissolved silver (top x-axis) during dealloying in potentiostatic (red) and galvanostatic (blue) modes. Reference: K. Ma, J.S. Corsi, J. Fu and E. Detsi, ACS Applied Nano Materials 1, 2, 2018[/caption]
Nanoporous metals exhibit an irreversible volume shrinkage during their formation by dealloying, the origin of which remains obscure. In this project, we use electrochemical dilatometry techniques to measure the irreversible shrinkage in nanoporous Au in situ during its formation by selective removal of Ag from Au-Ag alloys. A linear contraction up to ~9 % was recorded as illustrated in Figure 2. To identify the origin of this dimensional change, we borrow the time-dependent isothermal shrinkage model from sintering theory, which we use to fit the dimensional changes measured in our nanoporous Au during dealloying. This shrinkage model suggests that bulk transport through plastic flow is the primary mass transport mechanism responsible for the material contraction in dealloying. Based on the current understanding of the mechanism of porosity formation in dealloying, mass transport through surface diffusion of undissolved materials is critical in the process. The present work sheds new light in the sense that bulk transport through plastic flow seems also to play an important role in dealloying.
Reference: K. Ma, J.S. Corsi, J. Fu and E. Detsi, ACS Applied Nano Materials 1, 2, 2018
- Study of volume changes in Li-sulfur cathode during charging and discharging
Li-Sulfur (Li-S) cathodes suffer from volume changes arising during lithium (de)intercalation in the host materials, and during shuttling, which triggers the dissolution of the polysulfides into the electrolyte. The aim of this project is to study these volume changes during charging and discharging, using in-situ electrochemical dilatometry techniques, and use this information to elucidate the mechanism through which capacity fading occur in Li-S cathodes (see Figure 3).
Reference: M. Li, Z. Wang and E. Detsi, Unpublished
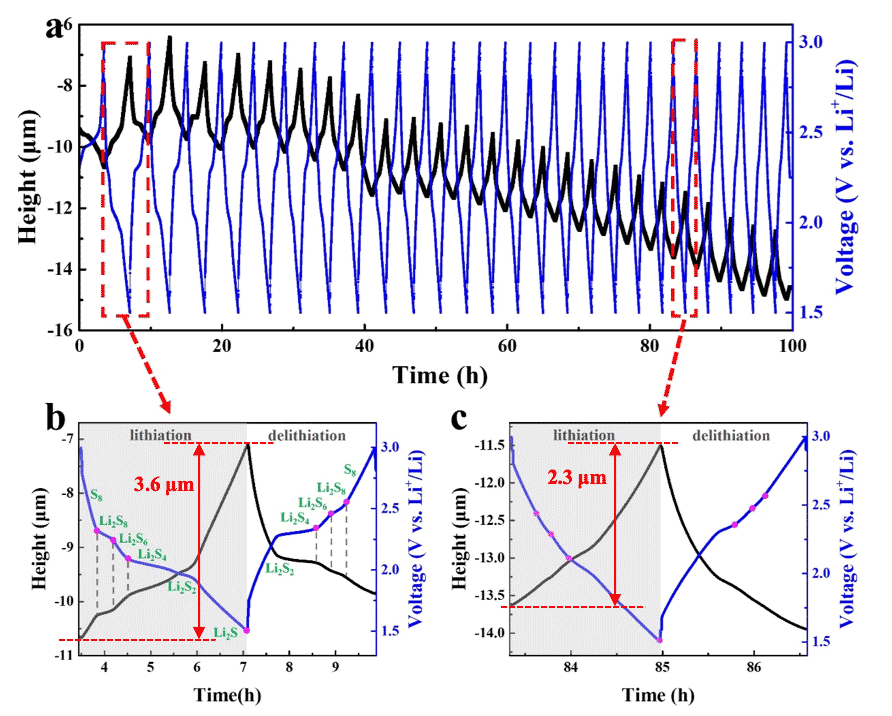 Figure 3: Volume expansion (black) and voltage (blue) of Li-S cathode during charging and discharging. Magnified data of the (b) 2nd and (c) 23th cycle. Reference: M. Li, Z. Wang and E. Detsi, Unpublished
Figure 3: Volume expansion (black) and voltage (blue) of Li-S cathode during charging and discharging. Magnified data of the (b) 2nd and (c) 23th cycle. Reference: M. Li, Z. Wang and E. Detsi, Unpublished